Cool Broad Deep: On Primary Water, Hydration, Metabolism
Abstract
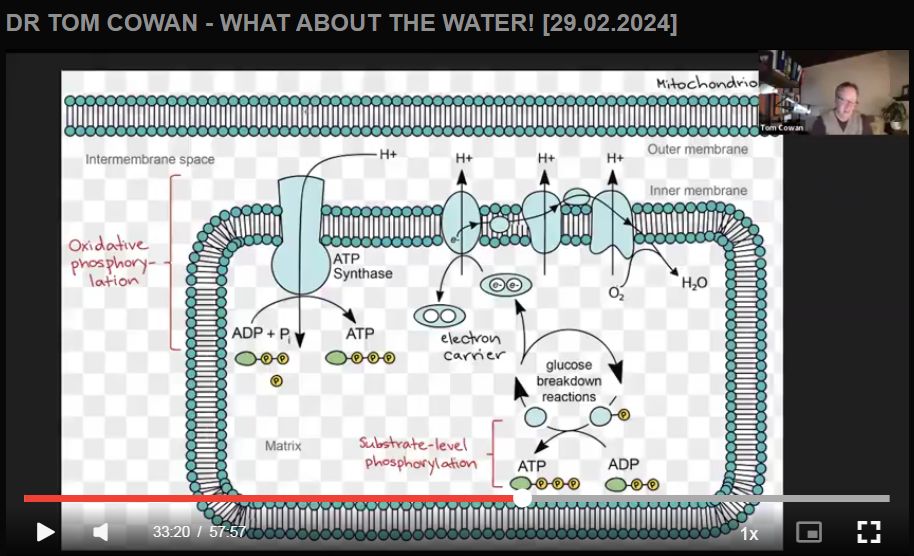
Dr Cowan explains how the cellular metabolism of food energy creates intra-cellular water that is “structured” into hydrogel form, what Dr Gerald Pollock has termed “EZ-Water”, and also how proper hydration is not merely bodily water intake but intra-cellular water creation. Dehyrdration, therefore, is actually a metabolic fault that is not fixed (but instead worsened, due to dilution of electrolytes) by simply drinking more water.
Primary water, well water, and natural spring water are terms that refer to different sources and types of groundwater. Understanding each one requires exploring how they are formed, accessed, and utilized.

Primary Water
Primary water refers to water that originates deep within the Earth’s mantle, believed to be formed through the natural processes of the Earth’s geology, often involving the combination of hydrogen and oxygen atoms under high pressure and temperature. This type of water can emerge to the surface through geological activity, such as volcanic eruptions or the movement of tectonic plates, creating new water sources that have not been part of the Earth’s surface or atmospheric water cycle.
Primary water is thought to be a renewable resource, continually produced deep within the Earth, and is considered very pure due to its origin deep below the Earth’s contaminated surface layers. However, accessing primary water can be challenging and requires deep drilling, making it less common than other sources of water for human use.
Well Water
Well water is accessed by drilling a borehole or well into the ground to tap into underground aquifers, which are layers of water-bearing permeable rock, sand, or gravel. The water in these aquifers is part of the Earth’s natural water cycle, having filtered down through the soil and rock from precipitation like rain and snow. Well water is a common source of freshwater for many rural and suburban areas.
The quality of well water can vary significantly depending on the local geology, the depth of the well, and the presence of contaminants in the area. Well water is usually tested regularly for contaminants such as bacteria, nitrates, and various chemicals to ensure it is safe for drinking. Depending on the results, treatment may be necessary to remove impurities.
Natural Spring Water
Natural spring water comes from natural springs, where water from underground aquifers naturally flows to the Earth’s surface. This process occurs when an aquifer is filled to the point that the water overflows onto the land surface or into a body of water like a lake or river. Spring water is often valued for its purity and mineral content, as it has naturally filtered through layers of rock and soil, which can imbue it with beneficial minerals and create a distinct taste.
Spring water is collected at the source or through a borehole that taps the underground formation feeding the spring. While generally clean due to natural filtration processes, spring water can still be susceptible to contamination depending on local environmental conditions and human activities. Like well water, it may require testing and treatment before consumption to ensure safety.
Conclusion
Primary water, well water, and natural spring water are all important sources of freshwater but differ in their origins, methods of access, and characteristics. Primary water comes from deep within the Earth and is not part of the surface water cycle, well water is accessed through drilling into aquifers, and natural spring water emerges naturally from the ground due to underground aquifers overflowing. Each type of water has its unique properties and uses, and understanding these differences is crucial for effective and sustainable water resource management.
https://www.bitchute.com/video/eTkM59yZmru2
EZ WATER
Exclusion Zone (EZ) water, also known as H3O2 or structured water, refers to a state of water that exhibits unique properties, different from regular H2O. This form of water is often associated with the term “cellular water” or “intra-cellular water” because it is believed to be the type of water found within the cells of living organisms.
Formation and Structure
EZ water is formed when water is in close contact with hydrophilic (water-loving) surfaces, such as cell membranes or certain types of polymers. The theory, primarily advocated by Dr. Gerald Pollack, suggests that this interaction causes the water molecules to reorganize into a more structured form, with a molecular formula of H3O2, making it more viscous, dense, and alkaline compared to H2O.
In this structured state, water molecules are believed to be arranged in a hexagonal lattice, similar to the crystalline structure of ice, but in a liquid form. This arrangement allows EZ water to create a separation of charge, with the EZ layer being negatively charged while the adjacent water (not in the structured state) becomes positively charged, creating a kind of battery that can store and release energy.
Properties and Functions
EZ water is thought to have several distinct properties:
– Increased Viscosity: It is thicker and more gel-like compared to bulk water.
– Exclusion of Solutes: EZ water tends to exclude particles and solutes, which is why it’s called “exclusion zone” water.
– Negative Charge: It typically carries a negative electrical charge, contributing to its potential role in cellular activities and energy transfer.
In biological systems, EZ water is believed to play a crucial role in cellular functions, such as:
– Cellular Hydration: Acting as a medium for cellular processes, EZ water may facilitate various biochemical reactions within cells.
– Energy Transfer: The structured nature of EZ water and its electrical properties might help in the energy transfer processes within and between cells.
– Protein Folding: The unique properties of EZ water are thought to assist in the proper folding of proteins, which is essential for their function.
Scientific Debate
The concept of EZ water, while supported by some experimental studies, remains a topic of debate within the scientific community. Critics argue that the evidence for the existence of H3O2 water under normal biological conditions is not conclusive, and further research is needed to fully understand its nature and implications.
Despite the debate, the study of EZ water and its potential roles in biological systems continues to be an area of interest due to its implications for understanding cellular hydration, bioenergetics, and the fundamental properties of water in living organisms.